Fertility
During the luteal phase of the menstrual cycle, which is the period after ovulation, the corpus luteum produces progesterone, an essential hormone to prepare the uterus for possible implantation and to support the onset of pregnancy.
After fertility treatment or in vitro fertilization (IVF), luteal phase support is essential to maximize the chances of successful embryo implantation and maintenance of pregnancy. Progesterone supplementation in women who have undergone fertility treatment or IVF is given after ovulation or embryo transfer to prepare the woman's uterus to receive the embryo. The aim is to create a favorable environment for implantation and continued pregnancy.
Because its chemical formula and spatial configuration are identical to the endogenous hormone produced by the ovaries (bio-identical), the progesterone contained in Utrogestan® is particularly suitable as a luteal phase supplement during in vitro fertilization (IVF) cycles 1-3.
Luteoplacental mismatch
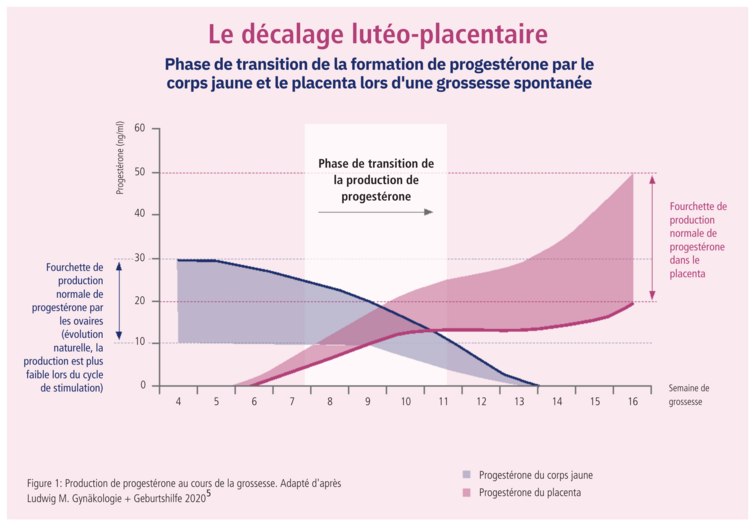
Luteo-placental mismatch, also known as utero-placental mismatch, refers to an imbalance in progesterone production between the corpus luteum (which forms after ovulation) and the placenta during pregnancy. This mismatch occurs when the corpus luteum produces progesterone, but the placenta is not yet fully functional and does not produce enough progesterone to sustain the pregnancy. This can lead to a drop in progesterone levels in the body, which can be worrying for the maintenance of the pregnancy, especially during the crucial first few weeks.
Early in pregnancy, luteal progesterone production increases. Later, around the 7th week of pregnancy, placental steroidogenesis begins to take over from ovarian steroidogenesis. At this point, there is a plateau or decrease in the concentration of progesterone in the blood. This process, known as "luteoplacental lag", is a critical phase that is associated with an increased risk of early miscarriage or pregnancy complications such as intrauterine growth retardation or premature birth.4
Women with luteo-placental mismatch may benefit from additional progesterone treatment to compensate for the hormonal imbalance and support the pregnancy. Medications such as Utrogestan®1 or other forms of progesterone can be used for this support.
It's important to note that luteo-placental mismatch isn't always the cause of pregnancy problems, and that other factors can also play a role. If a woman has concerns about her pregnancy or hormone levels, it's essential to consult a healthcare professional for a thorough assessment and appropriate treatment if necessary.
Luteal phase support after IVF treatment
External progesterone supplementation is the norm in the treatment of child desire,6 as luteal progesterone production after ovarian stimulation and follicular puncture may be disrupted or insufficient.7 This is why after embryo transfer, the woman continues to receive progesterone to support and promote adequate endometrial maturation and ensure the best chances of implantation and maintenance of pregnancy.8,9 If pregnancy is confirmed, progesterone can be administered during the first weeks of gestation, until the placenta begins to produce enough progesterone to support the pregnancy autonomously.
According to a survey of 284,600 IVF cycles per year, 408 centers and 82 countries, over 90% of luteal phase support treatments in IVF cycles involve vaginally administered progesterone - as monotherapy (77%) or in combination with i.m. progesterone (17%).6
Progesterone supplementation is also useful in the weak corpus luteum phase after pregnancy has been established, as the point at which endogenous steroid synthesis by the placenta is sufficient varies from woman to woman. To avoid abortions due to progesterone deficiency, progesterone should be administered until at least 7 weeks' gestation, but no later than 12 weeks' gestation.1 International practice is to administer external progesterone until 8-12 weeks' gestation,6 which may help reduce the risk of abortion due to luteal phase insufficiency.10

References
1. Utrogestan® professional information: www.swissmedicinfo.ch
2. Kuhl H. Pharmacology of Progestogens. J Reproduktionsmed Endokrinol 2011;8(Sonderheft 1):157-177.
3. Stanczyk FZ, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev 2013;34(2):171-208.
4. Broessner AB, et al. Endokrine Therapie in der Lutealphase und in der Frühschwangerschaft. Gynäkologische Endokrinologie und Reproduktionsmedizin. Frauenheilkunde up2date 2010; 4: 83-94
5. Ludwig M. Ist eine Supplementierung sinnvoll oder nicht? gynäkologie + geburtshilfe 2020; 25: 28-9
6. Vaisbuch E, et al. Lutealphase support in assisted reproduction treatment: real-life practices reported worldwide by an updated website-based survey. Reprod Biomed Online 2014; 28: 330-5
7. Ludwig M, Diedrich K. Evaluation of an optimal luteal phase support protocol in IVF. Acta Obstet Gynecol Scand 2001; 80: 452-66.
8. Yanushpolsky EH. Luteal phase support in in vitro fertilization. Semin Reprod Med 2015; 33: 118-27.
9. van der Linden M, et al. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015; 7: CD009154.
10. Ory S. Progesterone supplementation after oocyte retrieval: how long is it really needed? Fertil Steril 2012; 98: 812.




