Ferinject®
Gives energy back to life1
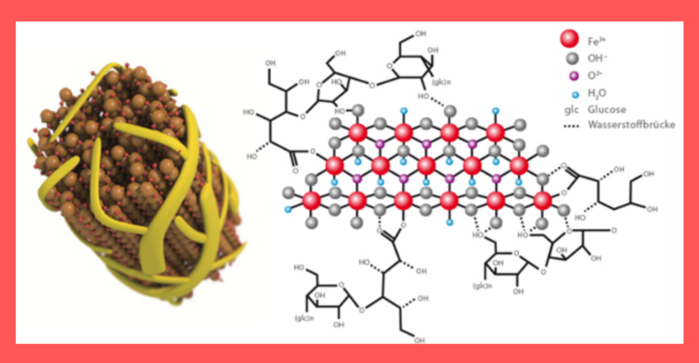
Ferric carboxymaltose (Ferinject® and Viyana®) is an iron-polymaltose solution consisting of a polynuclear core of iron (III) (oxyhydro)oxide stabilized by carboxymaltose.
Due to its stability, the complex does not release ionic iron under physiological conditions, and this limits the toxic effects of weakly bound iron.
In addition, the complex causes iron to be absorbed by macrophages and stored in the reticuloendothelial system of the liver, reducing the risk of large quantities of iron being present in serum. 2

Security of supply
We have a fully integrated supply chain in Switzerland, as well as a stock of active substances and iron ore several years old.3

Swiss Made
The active ingredient in Ferinject® was discovered in St. Gallen, where it is still produced by CSL Vifor. We employ 2,500 people in Switzerland and have over 70 years' experience in iron supply.

Experience
Since the launch of our first intravenous iron treatment in 1949, we have accumulated more than 25 million patient-years of experience with our products and innovative approaches for patients and physicians worldwide.4

Sustainability
We are aiming for a 40% reduction in our carbon emissions by 2030, and are focusing on minimizing total waste production through extraction, reduction and recycling.§,5

Services
In order to develop solutions that offer the greatest possible benefit to patients, we involve doctors and their staff in the process, organize training courses such as infusion courses, and encourage exchange.
Management
Ferinject® and Viyana® can be stored in original packaging at temperatures not exceeding 30°C. The maximum unit dose (1000 mg) can be administered in at least 15 minutes.9
Ferinject® and Viyana®
The same active ingredient for two distinct patient groups - Swiss Made in St. Gallen

Did you know?
In Switzerland, 22% of women are iron deficient. 6
Most of these women are below their annual deductible and must therefore pay for the necessary iron supplementation themselves.7
Around 78% of these patients have supplementary insurance 8 and can now benefit from Viyana®. 9,10
Viyana® - for your patients suffering from iron deficiency and fatigue * , with supplementary insurance. #,1,9
No entries available
Links & downloads
References
Area 1: Controlled by the company, e.g. emissions from combustion
in clean or controlled boilers, furnaces or vehicles.
Area 2: Emissions generated by one or more activities producing electricity, heating, cooling or steam.
heating, cooling or steam consumed by the plant, but which are not part of the plant.
* When oral iron treatment is insufficiently effective, ineffective or impossible.
# In many cases, the cost of iron deficiency treatment with Viyana® is covered by supplementary insurance. Information updated: January 2024.
1 Favrat B, et al. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron deficient women - PREFER a randomized, placebo-controlled study. PLoS One 2014;9(4):e94217.
2 Geisser P. The pharmacology and safety profile of ferric carboxymaltose (Ferinject®): structure/reactivity relationships of iron preparations. Port J Nephrol Hypert 2009:23(1):11-16
3 www.viforpharma.ch/life-at-vifor-pharma/behind-scenes-production-site-tour-st-gallen; Webseite zuletzt aufgerufen: März 2024.
4 Vifor Pharma annual report 2021, http://www.viforpharma.com/sites/vifor-corp/files/reports-andpresentations/vifor-pharma-annual-report-2021-full-version-en.pdf; last website accessed: March 2024 .
5 Annual report CSL 2023, https://investors.csl.com/annualreport/2023/; last website visit: March 2024.
6 Andersson M, Egli IM, Zimmerman MB. Eisenmangel. Schweizer Zeitschrift für Ernährungsmedizin 2010;(1):13-8.
7 Compulsory health insurance statistics, 2021 edition, May 2023.
8 www.comparis.ch/krankenkassen/zusatzversicherungen/spital-allgemeine-abteilung.
9 Professional information Ferinject®/Viyana®: www.swissmedicinfo.ch
10 Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs 2015 Jan;75(1):101-127.
Healthcare professionals may request a full copy of the clinical trial report from Vifor Pharma Switzerland.
Ferinject® and Viyana®. C: Carboxymaltose ferrique. I: Martial deficiency when treatment with oral iron is insufficiently effective, ineffective or impossible. Po: The total cumulative dose of Ferinject/Viyana must be calculated individually. Ferinject/Viyana can be administered by i.v. infusion (diluted in 0.9% NaCl) or i.v. injection (undiluted) in weekly doses of up to 1000 mg. CI: Hypersensitivity to the active substance or to one of the excipients, anemia without confirmed iron deficiency, iron overload, 1st trimester pregnancy. Precaution: Patients should be questioned before each administration of Ferinject/Viyana for AEs related to previous administration of i.v. iron preparations. Qualified medical personnel must be immediately available to assess and treat anaphylactic reactions. Administer only in a facility with full resuscitation facilities. Monitor patients for at least 30 min after administration, looking for signs and symptoms of a hypersensitivity reaction. Paraveinous administration may cause brown discoloration and should be avoided. Use with caution in acute or chronic infections, asthma or atopic allergies. Note sodium content of up to 5.5 mg/ml. Parenteral iron may cause hypophosphatemia, usually transient and without clinical symptoms. Isolated cases of hypophosphatemia requiring treatment have been reported in patients mainly with known risk factors, who have received a higher dose for a prolonged period. In case of high dose/long term treatment and risk factors, monitor for hypophosphatemic osteomalacia. Consult a physician in case of arthralgia or bone pain. G/A: CI during 1st trimester. Use during 2nd and 3rd trimesters only on strict indication. Fetal bradycardia may occur following a hypersensitivity reaction in the mother; the fetus should be monitored during administration. AEs: Common: hypophosphatemia, headache, facial flushing, dizziness, hypertension, nausea, injection/infusion site reactions. Occasional: immediate hypersensitivity reactions, paresthesias, tachycardia, hypotension, flushing, dyspnea, gastrointestinal disorders, dysgeusia, rash, pruritus, urticaria, erythema, myalgias, arthralgias, muscle cramps, fever, fatigue, peripheral edema, chills, pain, elevated AST, ALT, gamma-GT, LDH and ALP. IA: Simultaneous administration with oral iron preparations reduces absorption. Pres: 5 vials of 100 mg (2 ml) or 500 mg (10 ml), 1 vial of 500 mg (10 ml) or 1000 mg (20 ml). List B. Detailed information: www.swissmedicinfo.ch. Licensee: Vifor (International) Inc, CH-9001 St. Gallen. Distribution: Vifor Pharma Switzerland SA, CH-1752 Villars-sur-Glâne. Information updated: April 2022




